Abstract
Introduction
FL is characterized by the translocation t(14;18), resulting in constitutive overexpression of the key anti-apoptotic protein BCL2. Ven, a selective, potent oral inhibitor of BCL2, has shown profound efficacy combined with anti-CD20 antibodies in chronic lymphocytic leukemia and is in development for treating other hematologic malignancies, including non-Hodgkin lymphoma (NHL). Preclinical and early clinical data in indolent NHL suggest addition of Ven to R or chemo may improve efficacy. The open-label CONTRALTO study (NCT02187861) assessed efficacy and safety of a chemo-free regimen with Ven+R or randomized Ven + standard chemoimmunotherapy regimen BR vs BR for treatment of R/R FL.
Methods
Pts ≥18 yrs, with confirmed grade (Gr) 1-3a R/R FL, ≥1 line of prior therapy for lymphoma, and adequate hematologic and organ function with no history of B-refractory disease (chemo arms), were assigned at investigators' discretion to receive Ven+R or be randomized to BR +/- Ven, with stratification by 1 vs 0 GELF criteria and ≥ vs <12-mo duration of response (DOR) to prior treatment. The Ven+R arm comprised Ven 800mg daily for 1 yr + R (wk 1-4, mo 4, 6, 8, 10 and 12). After a safety run-in with Ven 600mg, pts in chemo arms were randomized 1:1 to Ven+BR (Ven 800mg daily for 1 yr+ 6 cycles standard BR) or BR. The primary endpoint was 6-mo (end of induction) complete metabolic response rate (CMR) assessed by Lugano 2014 criteria. Secondary endpoints included 1-yr CMR, progression-free survival (PFS), safety, and exploratory biomarker assessments. Pts were followed 6-monthly for safety and efficacy for up to 2 yrs. The final visit was March 15 2018.
Results
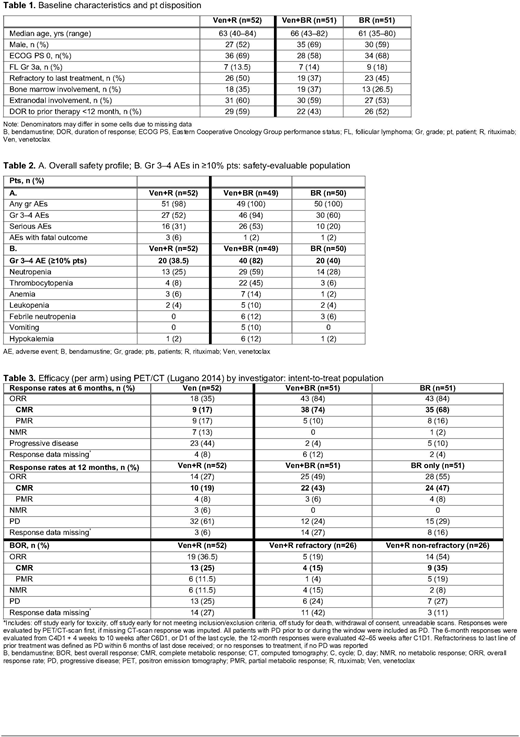
163 pts were enrolled; 52 received Ven+R, 9 in the safety run-in, 51 Ven+BR, 51 BR (Table 1). Median lines of prior treatment: 3 (1-6) in both Ven-treated arms and 2 (1-4) in BR arm. Ann Arbor stages III/IV: 88%, 73.5%, and 72.5% of pts in Ven+R, Ven+BR and BR arms, respectively. BCL2 expression by IHC and BCL2 translocations were observed in most pts (88% and 81% respectively); expression of MCL1 (1%) and BCLxL (29%) were low.
44 pts (90%) in Ven+BR arm required dose interruptions/modifications of any therapy compared with 21 (42%) in BR and 30 (58%) in Ven+R; most were of Ven: 20 (38.5%) in Ven+R arm and 43 (88%) in Ven+BR. 17 pts (35%) in Ven+BR arm and 2 (4%) in Ven+R discontinued Ven early. 30 pts (61%) in Ven+BR arm required dose interruption/modification of B versus 20 (40%) in BR; 10 pts (20%) in Ven+BR and 2 (4%) in BR discontinued B.
Neutropenia and thrombocytopenia were the most common Gr 3-4 adverse events (AEs) overall (Table 2B), and led to dose interruptions/modifications. They were highest in Ven+BR arm (59% neutropenia, 45% thrombocytopenia). Serious AEs were seen more frequently with Ven+BR (Table 2A) than Ven+R or BR; most were infection/infestation events: 22% Ven+BR, 11.5% Ven+R, 8% BR.
In Ven+R arm, 17% of pts reached CMR at 6 mo; 25% achieved CMR as best overall response (BOR), of whom all achieved minimal residual disease (MRD) negativity at mid-induction. A population of pts who were non-refractory to prior therapy had improved response (54% overall response rate, 35% CMR as BOR). Six-mo CMR rates were 74% and 68% with Ven+BR and BR, respectively, and were equal at 1 yr (Table 3). PFS analysis did not show a difference between these arms (adjusted HR, 0.69; p=0.2088). Although only 61.2% of pts in Ven+BR arm had ≥90% B dose intensity vs 95.8% in BR arm, efficacy in the 2 arms was comparable in CMR and PFS. Of note, pts maintaining all cycles of Ven+BR had a 6-mo CMR of 100%. Efficacy was comparable in biomarker subgroups, and did not identify clear predictors of response.
Conclusion
Ven+R had an acceptable toxicity in the population studied; this arm included a high number of pts with refractory status and shorter DOR to prior treatments, potentially leading to modest overall efficacy. Improved response rates and MRD negativity, and longer PFS were observed in a subgroup of pts with non-refractory FL, showing efficacy of Ven+R in FL. Addition of Ven to BR at the dose and schedule studied resulted in increased toxicity (Gr 3-4 cytopenias and gastrointestinal events), leading to dose interruptions/discontinuations, with consequent limitation of overall tolerability. However, despite lower dose intensity of BR in Ven+BR arm, efficacy remained the same between the 2 chemo-containing arms, mandating optimal dose delivery for improved efficacy in combination with chemo in future studies.
Zinzani:Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Honoraria, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Speakers Bureau. Flinn:Portola: Research Funding; Curis: Research Funding; Incyte: Research Funding; Genentech: Research Funding; Trillium: Research Funding; Takeda: Research Funding; Verastem: Consultancy, Research Funding; Forty Seven: Research Funding; Forma: Research Funding; Novartis: Research Funding; Kite: Research Funding; Celgene: Research Funding; ArQule: Research Funding; BeiGene: Research Funding; Infinity: Research Funding; Pharmacyclics: Research Funding; Verastem: Research Funding; Calithera: Research Funding; Agios: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding; Constellation: Research Funding; Pfizer: Research Funding; Gilead: Research Funding; TG Therapeutics: Research Funding; Janssen: Research Funding. Yuen:Seattle Genetics: Research Funding. Topp:F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Regeneron Pharmaceuticals, Inc.: Honoraria, Research Funding. Rusconi:Celgene: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria. Fleury:Novartis Pharmaceuticals Corporation: Consultancy; Merck: Consultancy; Janssen: Consultancy; Seattle Genetics: Consultancy; Gilead: Consultancy; Lundbeck: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy; Celgene: Consultancy. Pro:Takeda Pharmaceuticals: Honoraria, Other: Travel expenses; kiowa: Honoraria; Seattle Genetics: Consultancy, Other: Travel expenses, Research Funding; portola: Honoraria. Gritti:Autolus: Consultancy. Crump:F. Hoffmann-La Roche Ltd: Consultancy; Servier Canada: Consultancy; Jansen-Ortho: Consultancy. Samineni:Genentech Inc: Employment, Other: Ownership interests non-PLC. Sinha:F. Hoffmann-La Roche Ltd: Employment. Punnoose:Roche: Equity Ownership; Genentech Inc: Employment. Szafer-Glusman:F. Hoffmann-La Roche Ltd: Employment, Other: Ownership interests PLC. Danesi:F. Hoffmann-La Roche Ltd: Employment. Petrich:Abbvie: Employment, Other: Ownership interests PLC. Kornacker:F. Hoffmann-La Roche Ltd: Employment. Humphrey:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Mobasher:Genentech Inc: Employment; F. Hoffmann-La Roche Ltd: Other: Ownership interests non-PLC. Hiddemann:F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal